by user | Nov 16, 2023 | Uncategorized
Credit Steven B. Krivit
Tritium is a key component in most fusion research projects, with a significant portion of its limited availability earmarked for the International Thermonuclear Experimental Reactor (ITER) initiative. The process of generating tritium, which involves targeting Lithium-6 with neutrons, might appear simple in theory, yet it is fraught with numerous technical hurdles in practice. Currently, we do not know of any endeavors specifically aimed at producing tritium in quantities sufficient to sustain both experimental and future operational reactors. This seems to be a critical bottleneck that can negatively affect the public availability of fusion energy.
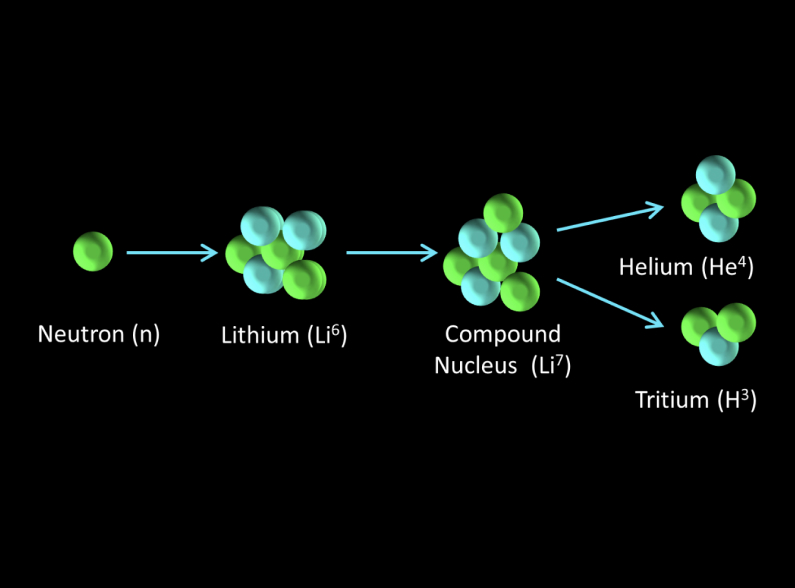
Applying a neutron beam to lithium results in nuclear reactions that can produce tritium, an isotope of hydrogen, among other products. The specific reactions depend on the isotope of lithium (either Lithium-6 or Lithium-7, which are the two stable isotopes of lithium) and the energy of the neutrons.
Neutron Interaction with Lithium-6:
When Lithium-6 (⁶Li) absorbs a neutron, it undergoes a nuclear reaction that produces tritium (³H) and helium-4 (⁴He). The reaction can be represented as follows:
⁶Li+n→³H+⁴He
⁶Li+n→³H+⁴He
This reaction is exothermic, releasing energy in the form of kinetic energy of the reaction products.
Neutron Interaction with Lithium-7:
When Lithium-7 (⁷Li) interacts with a neutron, especially a fast neutron, it can also produce tritium, but the reaction is different and less likely compared to Lithium-6. The reaction with Lithium-7 usually produces helium-4, a neutron, and tritium:
⁷Li+n→⁴He+³H+n
⁷Li+n→⁴He+³H+n
This reaction is less direct and less efficient for tritium production compared to the Lithium-6 reaction.
These reactions are particularly significant in the context of nuclear fusion, especially in fusion reactor designs that use deuterium-tritium fuel. In these reactors, Lithium is often used in the surrounding blanket material to absorb the high-energy neutrons produced during fusion reactions, thereby breeding Tritium, which is then used as fuel in the fusion process. This method is crucial for sustaining the fusion reaction, as natural tritium is scarce and must be replenished.
Additionally, the interaction of neutrons with lithium is also utilized in certain types of nuclear batteries, known as betavoltaics, where the beta particles (electrons) emitted during the decay of tritium are used to generate electrical power.

by user | Nov 4, 2023 | Uncategorized
Whether hydrogen fuel cell cars will replace battery electric vehicles (EVs) is mostly a matter of time and depends on several factors, including technological advancements, infrastructure development, environmental policies, and market preferences. Here are some points to consider:
1. Energy Efficiency: Battery electric vehicles are generally more energy-efficient than hydrogen fuel cell vehicles (CVs). In EVs, electricity stored in the battery is used directly to power the electric motors, while in CVs, energy losses occur during the production, storage, and conversion of hydrogen into electricity.
2. Infrastructure: EVs currently have an advantage because electric charging stations are more widespread than hydrogen refueling stations. Developing a hydrogen infrastructure is more costly and complex, involving the production, storage, and transportation of hydrogen.
3. Range and Refueling: Hydrogen cars have a longer range and can be refueled more quickly than most EVs, which can be a significant advantage. However, EV technology is rapidly advancing, with improvements in battery density and charging speed.
4. Environmental Impact: The environmental benefits of CVs depend on how the hydrogen is produced. Currently, most hydrogen is produced using fossil fuels, which negates some of the environmental advantages. When Fusion Energy becomes available CVs could become more attractive.
5. Technological Developments: Both EV and CV technologies are advancing. Developments such as solid-state batteries for EVs could lead to faster charging times and longer ranges. Similarly, advancements in hydrogen production and fuel cell efficiency could make CVs more competitive.
6. Cost: Currently, EVs benefit from lower production costs compared to CVs. As battery prices continue to fall, this advantage could be sustained until equivalent cost reductions are achieved in the production of hydrogen fuel cells.
7. Market Forces and Policies: Consumer preferences, government incentives, and environmental regulations will shape the market. Some regions may favor EVs due to their cleaner energy grids, while others may invest in hydrogen as part of a broader strategy to reduce carbon emissions.
8. Niche vs. Mainstream: It’s possible that hydrogen cars might not replace electric cars entirely but will find a niche in certain markets or applications. For example, hydrogen might be more suitable for heavy-duty transport where the long range and quick refueling are critical, while EVs might continue to dominate the passenger car sector for a longer time.
In conclusion, it is unlikely that hydrogen cars will entirely replace electric cars in the near future. Instead, we may see a diversification of the market where both technologies coexist and are used in different contexts based on their respective strengths and the evolution of the supporting infrastructure. The potential for hydrogen in larger vehicle segments, such as trucks and buses, or in regions with abundant renewable energy for hydrogen production, could be significant.

by user | Sep 28, 2023 | Uncategorized
As the world grapples with the growing challenges posed by climate change and the depletion of fossil fuel reserves, the quest for a sustainable, renewable, and abundant energy source has become more critical than ever. Fusion energy holds the potential to be the transformative answer to our energy needs. This article explores the promise of fusion energy, its benefits, and why it could be the inexhaustible power source of the future.
What is Fusion Energy?
Fusion energy is the process that powers the sun and stars, where two light atomic nuclei, typically isotopes of hydrogen, combine to form a heavier nucleus, releasing an immense amount of energy in the process. Unlike nuclear fission, which is currently used in nuclear power plants, fusion reactions produce little to no long-lived radioactive waste and are inherently safer.
Endless Fuel Supply
The primary fuel for fusion reactions is isotopes of hydrogen, such as deuterium and tritium, which are abundant in nature. Deuterium can be extracted from seawater, while tritium can be produced within the fusion reactor itself. With an essentially limitless supply of fuel, fusion energy stands in stark contrast to finite fossil fuels like coal, oil, and natural gas.
Safety and Environmental Benefits
Fusion reactions do not produce greenhouse gases or air pollutants, making fusion power inherently clean and environmentally friendly. Unlike traditional nuclear fission reactions, fusion energy does not generate long-lived radioactive waste, reducing concerns about nuclear waste disposal and storage. In the event of any operational issue, the reaction safely stops with no risk of a runaway chain reaction or meltdown, providing an extra layer of safety.
Reliable Energy Source
Fusion energy offers a constant and reliable power supply. It is not dependent on weather conditions, unlike solar and wind power, and it can provide a stable baseload energy source. With fusion, we can mitigate the intermittency issues associated with renewable energy sources and ensure a consistent and dependable electricity supply.
Research and Advancements
Although fusion has long been pursued as a viable energy solution, significant progress has been made in recent years. Advancements in fusion technologies and materials have led to the development of innovative reactor designs that are more efficient, compact, and cost-effective. International collaborations, such as ITER (International Thermonuclear Experimental Reactor), are driving this progress, bringing the world’s top scientists and engineers together to unlock the potential of fusion energy.
Economics of Fusion Energy
Critics have often cited the high costs of developing fusion reactors. While it is true that building and operating fusion power plants present significant challenges, once the technology matures, fusion energy has the potential to be economically competitive. The virtually limitless fuel supply and low operational costs can make fusion power a cost-effective solution in the long run, especially when compared to the dwindling resources and environmental impacts associated with fossil fuels.
Conclusion
Fusion energy represents a transformative shift in our approach to meeting global energy demands sustainably. With an inexhaustible fuel supply, minimal environmental impact, and the promise of safe and reliable power generation, fusion energy is poised to be the renewable, clean, and limitless energy resource of the future. Continued investment in research and development is key to unlocking the full potential of fusion energy and securing a brighter, more sustainable future for generations to come.

by user | Sep 28, 2023 | Uncategorized
Direct Air Capture (DAC) is an innovative technology that holds great promise in the fight against climate change. As the world grapples with the urgent need to reduce greenhouse gas emissions, DAC has emerged as a powerful tool to capture carbon dioxide (CO2) directly from the atmosphere. In this article, we will explore what DAC is and how it benefits the environment.
What is Direct Air Capture?
Direct Air Capture is a process that involves the extraction of CO2 directly from the ambient air using various chemical, physical, or biological methods. Once the CO2 is captured, it can either be stored underground (carbon sequestration) or used for different industrial applications like producing synthetic fuels, enhancing greenhouse cultivation, or creating building materials.
How Does Direct Air Capture Work?
There are several different methods for Direct Air Capture, but the most common ones involve the use of chemical sorbents or mechanical processes. Chemical sorbents work by chemically binding with CO2 molecules present in the air, which can later be heated to release the CO2 for storage or utilization. Mechanical processes use specialized materials that can selectively absorb CO2 when exposed to air and release it when the material is heated.
Benefits for the Environment:
1. Mitigation of Greenhouse Gas Emissions: The primary advantage of DAC is its ability to directly address the source of CO2 emissions, even those from dispersed sources such as vehicles or buildings. By capturing CO2 from the atmosphere, DAC can help offset emissions that are difficult to eliminate through traditional methods.
2. Climate Change Mitigation: Reducing the concentration of CO2 in the atmosphere is crucial for mitigating global warming and its associated impacts. Direct Air Capture, in combination with carbon sequestration, can play a vital role in achieving the ambitious climate goals set forth by various countries and international agreements.
3. Utilization of CO2: Instead of simply storing captured CO2 underground, some DAC processes can convert the captured CO2 into useful products, such as synthetic fuels or building materials. This way, DAC not only reduces emissions but also contributes to a circular economy by recycling CO2 for productive purposes.
4. Potential Negative Emissions: If the captured CO2 is permanently stored underground, DAC can achieve negative emissions, which means removing more CO2 from the atmosphere than it releases. This is a crucial pathway to meet the goals of net-zero or even carbon-negative emissions.
5. Scalability and Flexibility: Direct Air Capture can be deployed in various locations, making it highly scalable and adaptable to diverse geographic settings. This flexibility allows it to address specific emission hotspots or regions where alternative carbon reduction measures may be limited.
Energy requirements for Direct Air Capture
Direct Air Capture (DAC) does require a significant amount of energy to operate. The process of capturing CO2 directly from the atmosphere involves several steps, and each of these steps consumes energy. The amount of energy required depends on the specific DAC technology used, the scale of the operation, and the efficiency of the system.
Some factors that contribute to the energy requirements of DAC include:
1. Air Intake: Drawing air into the DAC system requires energy to power fans or other mechanisms that facilitate airflow through the capture apparatus.
2. CO2 Capture: The actual process of capturing CO2 from the ambient air using chemical sorbents or mechanical processes necessitates energy inputs. In chemical sorbent-based DAC, the regeneration of the sorbents (to release the captured CO2) often requires heat, which can be energy-intensive.
3. CO2 Separation: After capturing CO2, the DAC system needs to separate it from the sorbent material or the mechanical capturing component. Separation processes can also demand significant energy inputs.
4. Compression: To store or utilize the captured CO2 effectively, it needs to be compressed, which consumes additional energy.
The energy requirements of DAC have been a subject of research and development efforts, as reducing the energy intensity is essential for making this technology more sustainable and economically viable. Innovations in materials, system design, and process optimization are continuously being explored to enhance the energy efficiency of DAC.
One potential approach to reducing DAC’s energy consumption is integrating it with renewable energy sources. For example, utilizing Fusion Energy in the near future excess energy can help offset energy usage from conventional sources and reduce the overall carbon footprint of DAC operations.
While DAC does demand energy, its benefits lie in its potential to capture CO2 emissions directly from the atmosphere, which makes it feasible to address emissions from dispersed sources and achieve negative emissions in the long run. Despite its energy requirements, DAC remains an essential technology in the arsenal of climate change mitigation strategies, especially when combined with sustainable renewable energy sources and other carbon reduction efforts.
Conclusion:
Direct Air Capture represents a significant advancement in the fight against climate change, offering a promising solution to reduce greenhouse gas emissions directly from the atmosphere. By combining innovation, scalable technology, and the potential for negative emissions, DAC can contribute substantially to meeting global climate targets and safeguarding the environment for future generations. However, it should be noted that DAC is not a standalone solution and should be accompanied by efforts to transition to renewable energy sources and implement sustainable practices to achieve a truly sustainable future.




